
By Wade Colburn, Product Manager – Cochlear Implants
As a company that is committed to quality both in our products and reporting, we are excited about the compliance to a new standard for cochlear implants.
What is ANSI/AAMI CI86:2017?
AAMI standards are common in the medical field with the goal of advancing safety in health technology. Cochlear Implants are one of the latest technologies to have a standard created, AANSI/AAMI CI86: 2017 Cochlear implant systems: requirements for safety, functional verification, labeling and reliability reporting.
ANSI/AAMI CI86: 2017 was developed by the AAMI Cochlear Implant Committee and establishes minimum safety and performance requirements for cochlear implants based on industry-accepted test methods. The intent of the standard is to assist manufacturers in testing and labeling the devices and in reporting device system reliability to the professional health care community and public.
While this standard specifies specific requirements, test procedures and labeling that should be followed, it also provides greater specificity on how both implant and processor reliability should be reported. Therefore, we will soon be publishing a reliability report in compliance with ANSI/AAMI CI86: 2017. This means we will have two different reliability reports available: European Consensus Statement reliability report and the ANSI/AAMI CI86:2017 reliability report.
How do these reports differ?
There are a few key differences between the two reports:
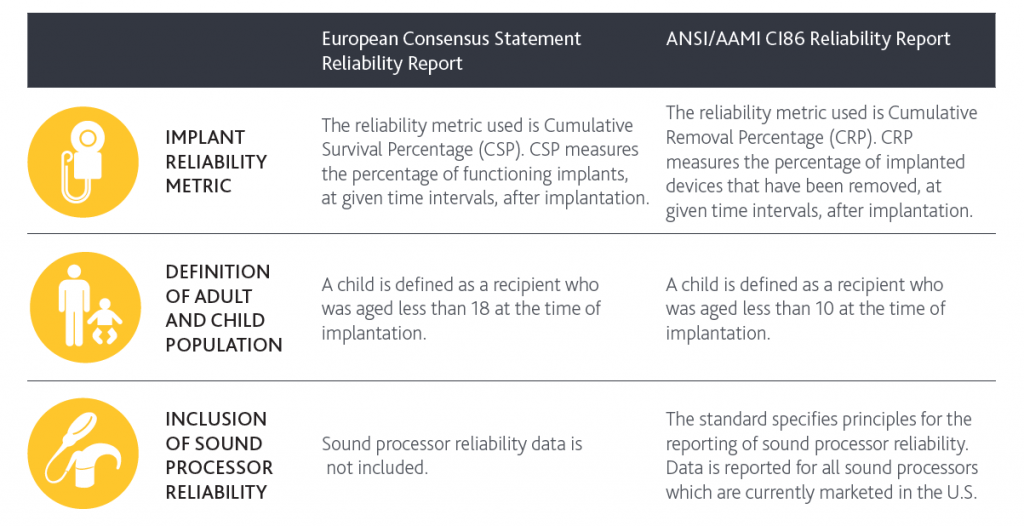
What is the difference in what is counted for implant reliability?
The European Consensus Statement report considers all device failures when reporting implant reliability. In contrast, ANSI/AAMI CI86 report considers device removals for any medical or device related reason. As an example, if an implant is removed because of an infection even if the device is still functioning, it will be reported as a failure with the new ANSI/AAMI CI86 standard, but it would not have been counted as part of the European Consensus Statement.
What should I expect regarding the sound processor reliability report?
Sound processor reporting will use Failed Component Return Rate (FCRR) as the metric to be reported. The FCRR is a percentage calculated by comparing the number of failed sound processors returned within a month to the cumulative sales of the same sound processor by the end of that month. The FCRR is reported as a monthly figure over a period of 24 months.
Check out this example (not real numbers):

When comparing sound processor reliability, consider these factors:
- Compare the FCRR over time: Monthly processor return volumes are variable and can be impacted by factors such as seasonality or how long the device has been available in the market. By considering the full 24 months of FCRR data, rather than individual months, you will gain a better view of overall processor reliability.
- Consider product generations: Predictors of manufacturer reliability would include both a consistent record of sound processor reliability and improving FCRR data for each new generation of sound processors.
To learn more about reliability reporting and download our latest reliability report in compliance with ANSI/AAMI CI86. For more information on the difference between the European Consensus Report and ANSI/AAMI CI86, click here .


