On September 26, 2022, CMS published a Decision Memorandum detailing changes in cochlear implantation coverage for Medicare beneficiaries. The policy change reflects an expansion of coverage under National Coverage Determination (NCD) 50.3 by which Medicare beneficiaries will be eligible for coverage at a level of hearing loss more closely aligned with FDA indications than prior to the changes. The changes to the coverage policy, detailed here, include lowering the threshold of one of the key eligibility requirements, “limited benefit from amplification” as defined by patient scores on recorded tests of open-set sentence recognition in the best-aided listening condition. Through that change, Medicare has broadened coverage to include individuals with test scores greater than 40% to less than or equal to 60%. We applaud the efforts of the primary investigators Teresa A. Zwolan, Ph.D., then Professor, Department of Otolaryngology, Head & Neck Surgery, Director, Cochlear Implant Program, Michigan Medicine and Craig A. Buchman, MD, FACS, Lindburg Professor and Chair, Department of Otolaryngology-Head & Neck Surgery, Washington University School of Medicine who both designed and executed the American Cochlear Implant Alliance sponsored Evaluation of Revised Indications (ERID) for Cochlear Implant Candidacy for the Adult CMS Population study which paved the way to meeting Medicare’s evidentiary requirements and who formally initiated the coverage change process with Medicare. Furthermore, we commend CMS on their efforts to improve access to cochlear implants for Medicare beneficiaries and are excited for what this means for Medicare beneficiaries. Medicare’s full coverage criteria, effective September 26, 2022 are as follows:
50.3 – Cochlear Implantation
A. General
A cochlear implant device is an electronic instrument, part of which is implanted surgically to stimulate auditory nerve fibers, and part of which is worn or carried by the individual to capture, analyze, and code sound. Cochlear implant devices are available in single-channel and multi-channel models. The purpose of implanting the device is to provide awareness and identification of sounds and to facilitate communication for persons who are moderately to profoundly hearing impaired.
B. Nationally Covered Indications
Effective for services performed on or after September 26, 2022, cochlear implantation may be covered for treatment of bilateral pre- or post-linguistic, sensorineural, moderate-to-profound hearing loss in individuals who demonstrate limited benefit from amplification. Limited benefit from amplification is defined by test scores of less than or equal to 60% correct in the best-aided listening condition on recorded tests of open-set sentence recognition. Patients must meet all of the following criteria.
- Diagnosis of bilateral moderate-to-profound sensorineural hearing impairment with limited benefit from appropriate hearing (or vibrotactile) aids;
- Cognitive ability to use auditory clues and a willingness to undergo an extended program of rehabilitation;
- Freedom from middle ear infection, an accessible cochlear lumen that is structurally suited to implantation, and freedom from lesions in the auditory nerve and acoustic areas of the central nervous system;
- No contraindications to surgery; and
- The device must be used in accordance with Food and Drug Administration (FDA)-approved labeling.
C. Nationally Non-Covered Indications
Medicare beneficiaries not meeting all of the coverage criteria for cochlear implantation listed in Section B are deemed not eligible for Medicare coverage except as described in Section D below.
D. Other
CMS may provide coverage of cochlear implants for beneficiaries not meeting the coverage criteria listed in Section B when performed in the context of FDA-approved category B investigational device exemption clinical trials as defined at 42 CFR 405.201 or as a routine cost in clinical trials under section 310.1 of the National Coverage Determinations Manual titled Routine Costs in Clinical Trials.
Prior to the recent decision, CMS criteria defined limited benefit from amplification by test scores of less than or equal to 40% correct in the best-aided listening condition on tape-recorded tests of open-set sentence recognition. The prior policy also allowed for coverage of candidates who score greater than 40% and less than or equal to 60% when the provider was participating in an approved clinical trial. The clinical trial requirement now goes away, as does the outdated “tape-recorded” requirement for presenting tests.
What does this Mean for Medicare Beneficiaries?
Jim Byrd, Senior Director, Reimbursement Strategy at Cochlear, had the opportunity to speak with Dr. Terry Zwolan who, together with input from Dr. Craig Buchman, shared the following insights and what this means for Medicare beneficiaries.
Jim: Dr. Zwolan, when you and I were talking, you immediately thought of patients who could now benefit. What does this mean for existing candidates who may have not qualified under the prior Medicare criteria and new candidates coming into a practice going forward?
Dr. Zwolan: Yes, this certainly does bring to mind several patients. Previously, it was difficult to tell a patient that they were a candidate for a cochlear implant (i.e. they met FDA indications), but they could not receive one since their insurer would not support a cochlear implant for them. This was because their insurer, meaning Medicare, had stricter requirements than most private insurers, and that Medicare’s indications were even stricter than the ones approved by the FDA. This recent expansion feels so right, because it no longer places Medicare beneficiaries at a disadvantage. Importantly, this expansion will enable patients with Medicare to receive an implant sooner, which we know is important. Richard Dowell published a study in 20161 where he demonstrated that recipients’ chances of a good outcome are significantly better if implantation occurs relatively soon after the onset of severe hearing loss and before the loss of all functional auditory skills. Thus, there is a very good chance that this change will positively impact outcomes as many patients will now be able to receive implants in a timelier manner.
Jim: Does this change anything you may do in the way of qualifying cochlear implant candidates?
Dr. Zwolan: We do not think this expansion will have great impact on the tests used to determine candidacy for a CI, per se, since many clinics use similar test measures to evaluate candidacy, regardless of the patient’s insurer. Such test batteries often include more than just a sentence test, such as word tests or questionnaires, to receive a comprehensive picture of how a patient’s hearing loss impacts their daily life. In audiology, we typically refer to this as the cross-check principle, where we use more than one test to ensure the results we are obtaining are all pointing to the same conclusion.
It’s important to note that clinicians have experience testing and recommending CIs for patients who obtain preoperative sentence scores in CMS’ recently expanded range of 40-60%. This is because FDA indications have included adults with sentence scores that fall within this range for several years. An important part of the CI evaluation process is the counseling that occurs following testing. Clinicians will continue to inform patients about the benefits of implantation, including the improvements in speech recognition and quality of life that are typically experienced by other CI recipients with similar case histories.
Jim: What would you say to a candidate that has previously been told they do not qualify?
Dr. Zwolan: We would be excited to connect with candidates who were previously told they do not qualify, particularly if their scores fell between 40 and 60% correct on sentences. We would explain to them that Medicare supported a national study to consider expansion of candidacy, and that the results of that study demonstrated that Medicare beneficiaries with scores similar to theirs clearly benefitted from a CI, so Medicare supported this change. We would encourage them to return to the clinic to have their candidacy re-evaluated and so we could discuss this potential next step in their journey to improved hearing. We would also encourage clinicians to contact Medicare beneficiaries who were previously denied a CI, even if their scores fell outside these new indications as it is possible that their hearing loss has progressed. With such patients, we would tell them we are not certain about their candidacy until they participate in testing, but it would be worthwhile for them to come in to be evaluated.
Jim: Any other thoughts?
Dr. Zwolan: We would like to applaud CMS and their decision to expand their indications for cochlear implants. This process helped me realize that the health and safety of seniors is an essential component of the decisions Medicare makes about coverage of medical procedures. Although this process took a long time and had many requirements, Medicare’s due diligence enabled us to perform a thorough study that provides strong evidence about the benefits of CIs for patients over the age of 65. Importantly, they recognize the value that improved hearing through cochlear implantation can bring to the lives of seniors, and for that we are very grateful. Finally, we would like to thank the many clinicians and patients that participated in the study and the process to approval of the new Final Rule and the administrative officials at CMS that made this possible.
Case Examples
Below are illustrations of two examples of candidates who would not have qualified for a cochlear implant under previous CMS guidance, but now do qualify for a CI.
| Case Example #1 |
History: 68-year-old female with progressive hearing loss beginning at age 45. Appropriately aided for last 12 years, recently experiencing more difficulty on the phone & particularly when communicating in noise.
Test Results: Testing included both CNC words and AzBio sentences administered in quiet and in a +10 signal to noise ratio to further evaluate her report of difficulty hearing in noise. CNC testing was used as a cross-check of sentences scores, helped evaluate differences between ears, and verified that each ear is struggling to hear individual speech sounds (she’s missing 80% of speech sounds in quiet). AzBio sentence scores were required to determine if she met CMS indications, and provided important clinical information, validating the difficulty she reported in noise, and revealing that she’s missing more than 40% of connected speech in quiet. If this patient was seen prior to the expansion, she would not have qualified for a CI since her best aided score on sentences exceeded 40%. However, because indications have expanded, this patient qualifies for a CI both in quiet and in noise, It should be noted that CMS indications also require patients meet the FDA approved indications for the device selected for implantation.
Summary: Because sentence scores administered at +10 SNR are both less than 50% correct, either ear could be selected for implantation. In this case, a decision was made to implant this patient’s right(poorer) ear, with the plan to monitor bimodal performance and to consider future implantation of the left ear if appropriate.
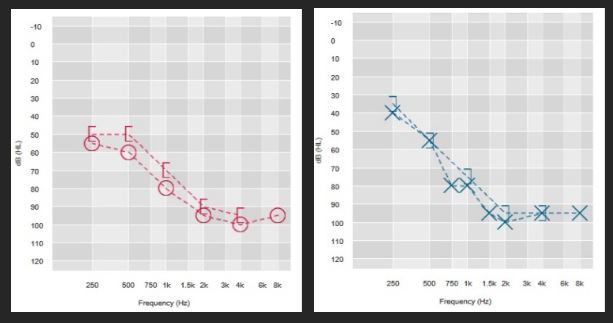
| Right Aided | Left Aided | Binaural Aided | |
| AZBio (quiet) | 46% | 52% | 56% |
| CNC Words | 12% | 20% | 28% |
| AZBio +10 | 20% | 32% | 42% |
| Case Example #2 |
History: 70-year-old male, rapidly progressing hearing loss that was first identified 5 years ago. Unable to communicate without visual cues and fully reliant upon captions for television watching. Hearing aids fit to real ear targets with minimal benefit (see results below).
Summary: If seen prior to expansion of CMS indications, candidacy for a CI would have been based upon clinical judgement and this clinic’s test protocol. If this clinic based candidacy on the best aided score obtained in quiet, the patient would not have qualified for a CI since his best aided sentence score exceeded 40% in quiet. If they based their decision on the AzBio scores obtained at +10 SNR, with additional consideration of the CNC scores for each ear, he would have qualified for a CI because his best aided AzBio score at +10 SNR was less than 40% correct. With the expanded indications, this patient qualifies both in quiet and in noise. This is an example of a patient that may have been turned away for a CI, despite his right ear having a profound hearing loss with no useable speech recognition. It also exemplifies the important role clinical judgement plays in determining candidacy for a CI.
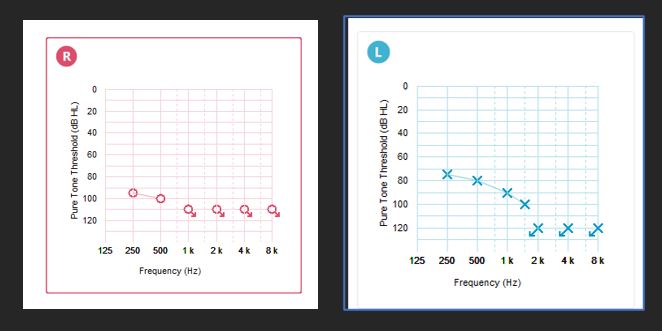
| Right Aided | Left Aided | Binaural Aided | |
| AZ Bio (quiet) | DNT | DNT | 48% |
| CNC Words | 0% | 32% | 28% |
| AZ Bio (+10 SNR) | 10% | 28% | 32% |
References
- Dowell, Richard C. (2016) The case for earlier cochlear implantation in postlingually deaf adults. Int J Audiology; 55 Suppl 2:S51-6.
Disclaimer: This material is intended for health professionals. If you are a consumer, please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information.


