By Ryan Lopez, Director, Nucleus® Product Management & Marketing at Cochlear Americas
Quality products start with Cochlear’s commitment to putting recipients first and expanding access to information to reinforce transparency. Longevity is an important factor when choosing a cochlear implant, especially if choosing for a child. High implant reliability can mean greater recipient satisfaction and less risk of additional surgery. When considering a cochlear implant, the latest data on short and long term reliability, including success and failure rates for both adults and children, should be accessible. Further, the durability of a sound processor is also important to ensure the entire cochlear implant system works as intended.
With the recent release of Volume 18 of the Nucleus Annual Reliability Report, we want to make sure the respective data and resources are provided to make informed decisions. Here’s how we report:
Implant reliability data
The implant data in this report is based on the reporting methodology recommended by International Standard ISO 5841-22,3, the reporting principles outlined in the European Consensus Statement on Cochlear Implant Failures and Explantations and expert recommendations from the International Classification of Reliability for Implanted Cochlear Implant Receiver Stimulators. This report meets the standards for cochlear implant reliability reporting outlined in these standards.

We also refer to implant reliability data based on the reporting standards and methodology recommended by ANSI/AAMI CI86 – Cochlear implant systems: Requirements for safety, functional verification, labeling and reliability reporting.
Sound processor reliability data
The sound processor data in this report meets the reporting standards and methodology recommended by ANSI/AAMI CI86 – Cochlear implant systems: Requirements for safety, functional verification, labeling and reliability reporting.
Reliability results
Cochlear’s latest implant, the Profile™ Plus Series, builds on the industry-leading thinness of the Profile Series Implant and provides access to MRI at 1.5 Tesla and 3.0 Tesla without removing the internal magnet.1
Commercially released in 2019, the Profile Plus Series Implant has delivered 99.97% reliability within one year, with 100% reliability for children within one year.2
Profile Series Implants
At only 3.9 mm, the Profile Series Implant was commercially released in 2014 as the thinnest cochlear implant in the world.3-4
The Profile Series Implant sets a new standard in implant reliability with a 99.81% combined Cumulative Survival Percentage within six years.2
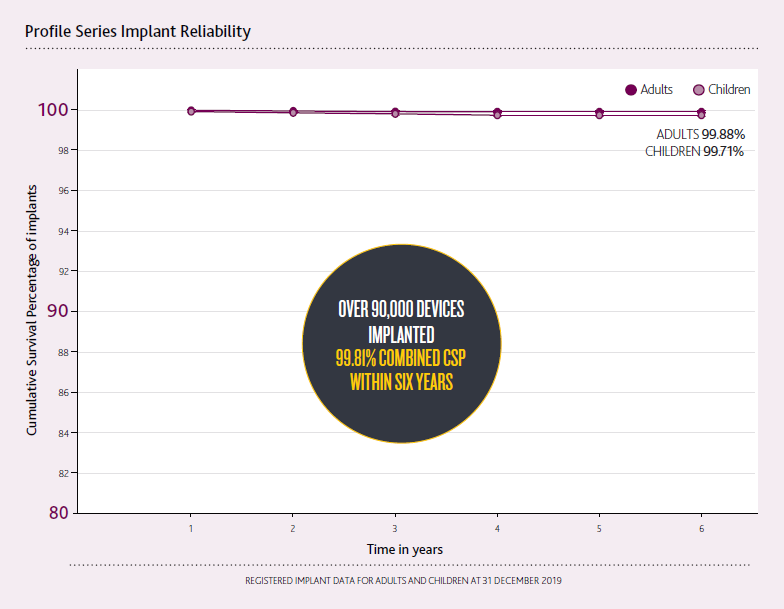
Confidence intervals smaller than 0.1% may not be clearly visible in the graphs.
CSP includes both device and accident-related issues.
Nucleus 7 Sound Processor
Released in 2017, the Nucleus 7 Sound Processor is our smallest and lightest behind-the-ear sound processor offering world-first connectivity and control directly from a compatible smartphone.* The Nucleus 7 demonstrates a Failed Component Return Rate (FCRR) of 0.3% over 22 months.2 This means if there were 10,000 Nucleus 7 devices to date, only 30 would be returned for repair each month.2
Quality drives Cochlear as the leader in implantable hearing solutions, connecting hundreds of thousands of people globally to a life full of hearing. We’re transforming the way people understand and treat hearing loss, and we’re committed to reaching more people to provide support for a lifetime of hearing.
To learn more, read the latest reliability report and watch this TECH Talk.
References:
- MRI Guidelines D774756.
- Cochlear Limited. D1712187 ISS1 MAR20. Cochlear™ Nucleus® Implant Reliability Report Volume 18, December 2019.
- Cochlear Nuclues Profile—CI512 Implant Spec Sheet. Technical Specifications. HiResolution™ Bionic Ear System by Advanced Bionics, [PDF-Internet] [cited 2015 Jan 5]. Available from: https://www.shablulim.com/wp-content/uploads/2013/11/028-M129-03_CISystemTechSpecs-Brochure.pdf.
- Concerto. World’s Smallest & Lightest Titanium Implant, [PDF-Internet] [cited 2015 Jan 5]. Available from: https://www.medel.com/data/pdf/ 22676.pdf.
*The Cochlear Nucleus 7 Sound Processor is compatible with Apple® and Android™ devices, for compatibility information visit www.cochlear.com/compatibility.
App Store is a service mark of Apple Inc., registered in the U.S. and other countries.
Android and Google Play are registered trademarks of Google Inc.