Bone conduction solutions are a well-established treatment option for patients with conductive hearing loss, mixed conductive-sensorineural hearing loss, and single-sided deafness. Options such as the Cochlear™ Osia® System*, Baha® Start or Baha® System** are predictable and cost-effective solutions that provide excellent hearing outcomes.1 With the introduction of Cochlear’s Candidacy, Evaluation and Fitting Protocol for Bone Conduction Solutions, clinics looking to begin or expand treating patients with a bone conduction solution, like the Osia System, have a roadmap to help them establish a consistent and evidence-based treatment practice.2
The Osia System revolutionized clinical treatment for patients by applying digital piezoelectric stimulation to become the world’s first active osseointegrated steady-state implant (OSI). The Osia System first received FDA approval in December 2019, and Cochlear recently celebrated the 10,000th Osia System recipient!
Incorporating bone conduction solutions into clinical treatment protocols
Justyn Pisa, AuD, and the team at the Surgical Hearing Implant Program in Manitoba provided insights into integrating bone conduction solutions into a medical portfolio of treatment options.
“Bone conduction options are part of our treatment course for conductive or mixed hearing losses (provided bone conduction thresholds are within acceptable limits).

We do prefer to see bone conduction thresholds of 45 dB or better in mixed losses, but not every potential candidate presents with ideal circumstances, so we try to match them with the best potential option on a case-by-case basis.
In general, we have our patients trial conventional amplification prior to receiving a bone conduction solution. However, in cases where hearing aids are not an option, we will look at a bone conduction solution first, either on a headband or implant.

We have fairly good communication between surgeons and audiologists on new referrals and potential candidates prior to surgery. In many cases, new patients are referred for assessment by our surgeon Dr. Garber or other public health audiologists in Canada who encounter patients with long-standing/permanent conductive hearing loss.
I try to ensure everyone is up to date on new candidates and will assist the scheduling clerk on medical assessment appointments once they’ve been cleared by audiology.

We have begun quarterly team meetings to review program needs, new technology, and our wait list for surgery. We generally use this time to discuss specific patients and potential outcomes with different implant options.”
Clinics looking to start offering Osia or Baha Systems into their treatment protocols, just like Justyn Pisa, AuD, and the Surgical Hearing Implant Program in Manitoba have done, have several clinical resources available to help them get started. These include Cochlear’s Candidacy, Evaluation, and Fitting Protocol for Bone Conduction Solutions, as well as including Otologic Management Services Insurance Support, our expert insurance and reimbursement team.
References
- Evans AK, Kazahaya K. Canal atresia: “Surgery or implantable hearing devices? The experts question is revisited” Journal of Pediatric Otorhinolaryngology 2007; 71, 367-374
- OSI140 ISS1 FEB23 Bone Conduction Protocol Whitepaper
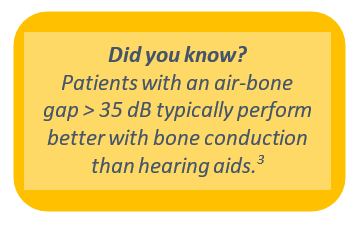
- de Wolf MJ, Hendrix S, Cremers CW, Snik AF. Better performance with bone‐anchored hearing aid than acoustic devices in patients with severe air‐bone gap. Laryngoscope. 2011;121:613-616.
*In the United States, the Osia 2 System is cleared for children ages twelve and older. In Canada, the Osia 2 System is approved for children ages five and older
**In the United States and Canada, the placement of a bone-anchored implant is contraindicated in children below the age of five.
***In the United States and Canada, the Nucleus System for single-sided deafness is approved for children ages five and older.