Assessment of Speech Understanding After Cochlear Implantation in Adult Hearing Aid Users
A Nonrandomized Controlled Trial
Buchman CA, Herzog JA, McJunkin JL, et al.
Hearing and Quality-of-Life Outcomes After Cochlear Implantation in Adult Hearing Aid Users 65 Years or Older
A Secondary Analysis of a Nonrandomized Clinical Trial
Wick CC, Kallogjeri D, McJunkin JL, et al.
Purpose
To measure speech understanding in quiet, speech understanding in noise, and health utility using a cochlear implant and hearing aid (bimodal hearing) in 96 subjects versus their pre-operative condition of two hearing aids (HAs).
Key messages
Significant improvement was seen at six months post-surgery in the areas of speech perception in quiet, speech perception in noise, perceived health utility and subjective assessment of disability in subjects with and without mild cognitive impairment. Of interest, the clinically meaningful audiometric and quality of life (QoL) benefits demonstrated by older adults suggest that cochlear implantation may facilitate healthy aging.
Methods
- Non-randomized controlled trial, multicenter, prospective, repeated measures design
- 13 clinical sites
- 100 subjects age 18 years and older with postlinguistic onset of bilateral moderate sloping to profound sensorineural hearing loss with an aided CNC word score of ≤40% in the ear to be implanted and ≤50% in the contralateral ear
Subjects were experienced HA users and completed objective speech perception testing and subjective QoL surveys prior to implantation. All enrolled subjects were implanted with a CI532 electrode, and used a Nucleus® 7 Sound Processor on the implanted ear and a new ReSound hearing aid on the contralateral ear. Subjects were seen by their clinicians at 1m, 3m, 6m and 12m post-activation, with endpoint measurements collected at the 6m visit. Subjects also completed a post-operative high-resolution CT scan to confirm electrode placement.
Results
Ninety-six subjects completed the six-month endpoint visit. Three serious adverse events requiring revision surgery occurred and all resolved without sequelae. By six months after activation, the average pre-operative CNC word score of 14.6% more than quadrupled to 60.9% in the implanted ear (change of 46.3 percentage points), and AzBio sentence scores at +10 SNR improved from 14.8% to 42.7% (change of 27.9 percentage points). In the binaural condition (N=94) CNC word scores increased from 28.8% with HAs to 69.2% using the bimodal solution of the Nucleus 7 and Resound hearing aid together (change of 40.5 percentage points), and the average AzBio sentences +10 SNR score of 31.8% rose to 55.9% (change of 24.1 percentage points) as shown in Figure 1.
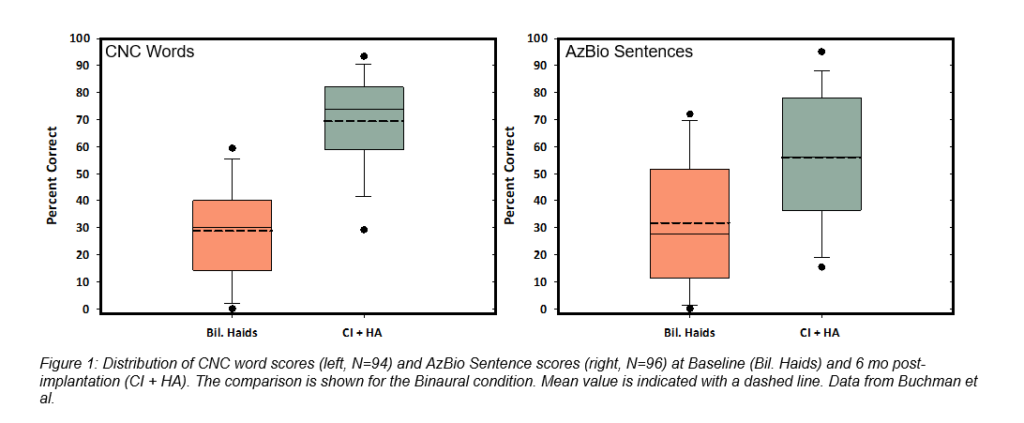
Statistically significant and clinically important improvements in the Health Utility Index 3 (HUI3) and the Speech, Spatial and Qualities of Sound survey (SSQ49) were evident at six months. The HUI3, which evaluates a variety of health attributes, saw the largest average score increase in the Hearing domain (.30) which directly contributed to a boost in the overall health-related QoL score from .46 to .64. (change of .18 points). Improvements were seen on the SSQ49 in all three subcategories, with the total score shifting from 3.2 using HAs to 5.7 using the bimodal solution (change of 2.5 points).
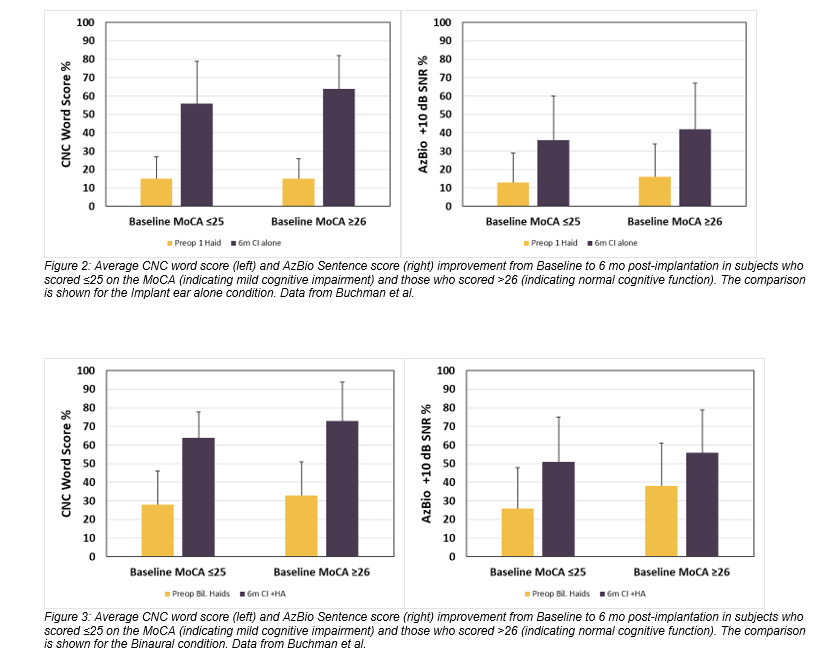
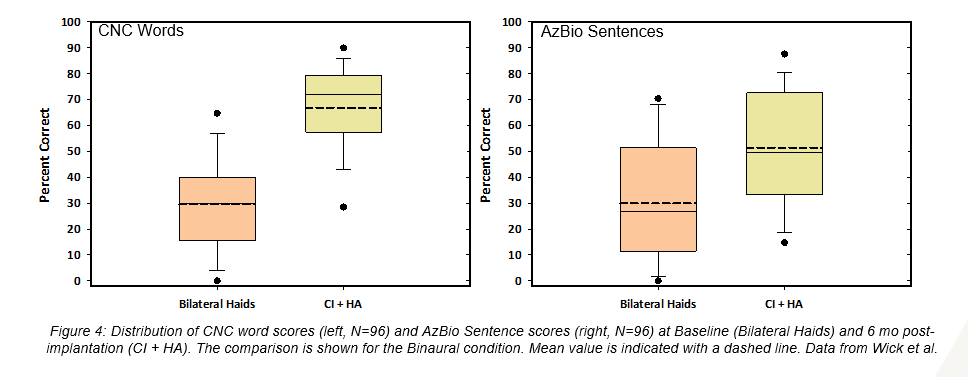
Eighty-one subjects were evaluated with the Montreal Cognitive Assessment (MoCA), and 48 of those subjects demonstrated mild cognitive impairment prior to implantation; however, as a group, the speech perception improvements of this cohort trended similarly to that of the entire study sample (Figure 2and Figure 3). Likewise, the subjects age 65+ (N=70) showed a substantial increase in performance with a score gain of 44.1 and 24.5 percentage points on CNC words and AzBio sentences +10 respectively in the implanted ear, and 37.2 and 21.6 percentage points in the bimodal condition (Figure 4). It is important to note that there were no major adverse events reported in this group, indicating a similar and acceptable risk profile to that of younger subjects.
Conclusions
Cochlear implantation surgery is safe and can greatly improve a person’s ability to understand speech in quiet and noise, thus improving QoL. These advantages were evident across the larger study group, as well as within the subgroups of those with mild cognitive impairment and those over the age of 65. The significant differences shown in performance and satisfaction scores when using a cochlear implant emphasize that individuals with moderate sloping to profound or worse sensorineural hearing loss should be offered a cochlear implant evaluation when their HAs are not providing enough benefit.
To learn more subscribe to Cochlear ProNews.
Citation:
- Buchman CA, Herzog JA, McJunkin JL, et al. Assessment of Speech Understanding After Cochlear Implantation in Adult Hearing Aid Users: A Nonrandomized Controlled Trial. JAMA Otolaryngol Head Neck Surg. Published online August 27, 2020. doi:10.1001/jamaoto.2020.1584
- Wick CC, Kallogjeri D, McJunkin JL, et al. Hearing and Quality-of-Life Outcomes After Cochlear Implantation in Adult Hearing Aid Users 65 Years or Older: A Secondary Analysis of a Nonrandomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. Published online August 27, 2020. doi:10.1001/jamaoto.2020.1585
Authors & Affiliations:
Department of Otolaryngology–Head and Neck Surgery, Washington University School of Medicine in St Louis, St Louis, Missouri
Craig A. Buchman, MD (1,2)
Jacques A. Herzog, MD (1,2)
Jonathan L. McJunkin, MD (1,2)
Cameron C. Wick, MD (1,2)
Nedim Durakovic, MD (1,2)
Jill B. Firszt, PhD (1,2)
Laura K. Holden, AuD (2)
Dorina Kallogjeri, MD, MPH (1,2)
Statistics Editor, JAMA Otolaryngology–Head & Neck Surgery
Dorina Kallogjeri, MD, MPH (1,2)
This article is intended to serve as a resource for clinicians to help keep up to date with current clinical literature and is intended for professionals only. Clinical literature is based on research, which may include the experimental use of new or currently available products and technologies. Therefore, literature presented on this blog may represent use of Cochlear products that does not align with the intended use or indications approved by regulatory bodies, also known as off-label use. Cochlear does not condone any off-label use of its products, and it is not Cochlear’s intent to promote off-label use by providing this blog as a resource to clinicians.
This content is meant for professional use. If you are a consumer, please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information. Views expressed are those of the individual. Consult your health professional to determine if you are a candidate for Cochlear technology.
All figures are our own.
For compatibility information and devices visit www.Cochlear.com/compatibility and resound.com/compatibility. For a list of compatible ReSound hearing aids visit www.Cochlear.com/nucleus/compatibility


