On January 10, 2022, the FDA approved an expansion of the Cochlear™ Nucleus® System indications to include Single Sided Deafness and Unilateral Hearing Loss (SSD/UHL) for individuals 5 years and older.
Every year, about 60,000 people in the United States acquire SSD. 1 UHL/SSD can impact an individual’s quality of life, including mental fatigue because of increased listening effort, as well as impacting understanding of speech in noise and localizing where sounds are coming from.2,3,4,5,6
Aligning with our company mission, a goal of the Cochlear Market Access team is to help more people hear by removing barriers to accessing implantable hearing solutions. We are pleased to announce a major milestone within the commercial payer landscape for CI for SSD/UHL:

Policy adoption begins with clinical providers who are willing to passionately advocate for coverage on behalf of their patients. Communicating to payers the need to expand coverage policies occurs in various ways not the least of which is the need that is emphasized with every prior authorization request, peer to peer conversation, and completed level of appeal. The collaborative effort of providers across the nation who embraced the cause emphasized an unmet need and accelerated the time from the Cochlear FDA approval to policy adoption at both the national and local levels. Below is a sample of the commercial payer landscape which recently expanded coverage to their members.*
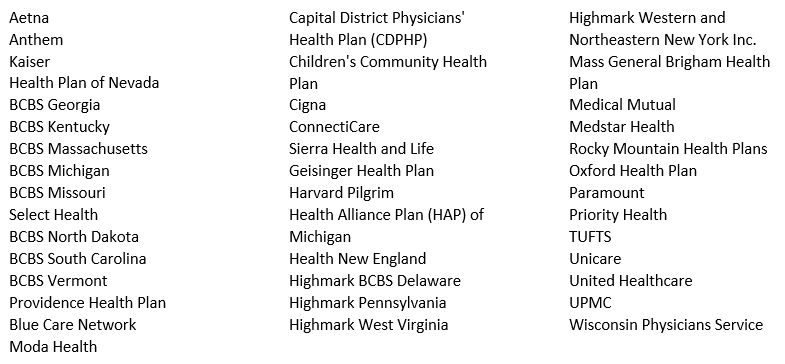
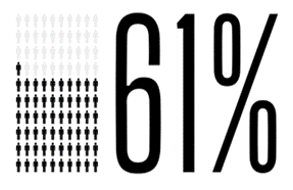
Reaching this milestone in coverage does not conclude our effort in closing the gap in coverage. Cochlear’s Market Access team invites and welcomes all providers to partner with us on the journey to help more people hear. Please visit the Reimbursement HUB for your local Regional Reimbursement Manager’s contact information and additional resources.
*Coverage may vary by specific plan. Providers and members should contact their insurance plans to determine coverage according to their benefit plan design.
Information provided by Cochlear Americas regarding insurance coverage or reimbursement is provided as guidance only and is not intended as reimbursement or legal advice. Cochlear Americas makes no representation or warranty regarding such information or its completeness, accuracy, fitness for a particular purpose, or that following such guidance will result in any form of coverage or reimbursement from any insurer. Information presented is subject to change at any time. To be sure that you have the most current and applicable information available for your unique circumstances, please consult your own experts and seek your own legal advice regarding your reimbursement needs. In all cases, products or services billed must be medically necessary, actually performed and appropriately documented in the medical record.
1. Weaver, J. (Web. 28 April 2017). “Single-Sided Deafness: Causes, and Solutions, Take Many Forms.”. Hearing Jornal, 68.3 (2015).
2. Buss E, Dillon MT, Rooth MA et al. Effects of Cochlear Implantation on Binaural Hearing in Adults with Unilateral Hearing Loss. Trends Hear 2018; 22:2331216518771173
3. Firszt JB, Reeder RM, Holden LK, Dwyer NY, Asymmetric Hearing Study T. Results in Adult Cochlear Implant Recipients with Varied Asymmetric Hearing: A Prospective Longitudinal Study of Speech Recognition, Localization, and Participant Report. Ear Hear 2018; 39:845-862.
4. Tavora-Vieira D, Rajan GP, Van de Heyning P, Mertens G. Evaluating the Long-Term Hearing Outcomes of cochlear Implant Users with Single-sided Deafness. Otol Neurotol 2019;40:e575-e580.
5. Dillon MT, Buss E, Anderson MLet al. Cochlear Implantation in Cases of Unilateral hearing loss: Initial Localization Abilities. Ear Hear 2017; 38-611-619.
6. Dillon MT, Buss E, Rooth MA et al. Effect of Cochlear Implantation on Quality of Life in Adults with Unilateral Hearing Loss. Audiol Neurootol 2017; 22:259-271.


