Cochlear™ Nucleus® implant (CI) candidacy and indications are important when counseling both adult and pediatric patients. In adults, cochlear implants can significantly improve hearing performance and quality of life.1 This can have a positive effect on the patients’ overall physical and mental health as well by reducing anxiety and stress.2
For pediatric patients, a cochlear implant is vital to giving children access to sound for proper speech and language development.3
Below you will find U.S. FDA indications regarding Nucleus cochlear implant candidacy for both adults and children. As well as Nucleus Hybrid™ System* candidacy.
For those over the age of 18 the criteria include:
Those who have bilateral, pre, peri or postlinguistic sensorineural hearing impairment.
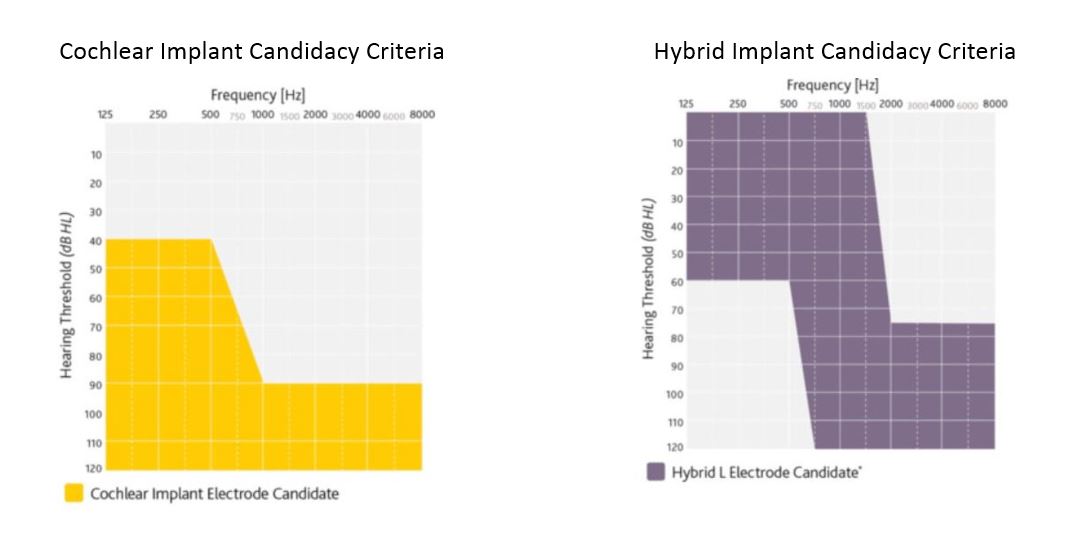
Those with a moderate to profound hearing loss in the low frequencies and profound (≥90 dB HL) hearing loss in the mid to high speech frequencies. Also,the candidate must obtain limited benefit from appropriate hearing aids. Limited benefit from amplification is defined by test scores of 50% correct or less in the ear to be implanted (60% or less in the best-aided listening condition) on recorded tests of open set sentence recognition.
The Cochlear Nucleus® Hybrid System is made up of the Nucleus Hybrid L24 implant and the Nucleus sound processor combining acoustic amplification for the low frequencies with electric stimulation for the high frequencies. The Nucleus Hybrid System is indicated for unilateral use in patients age 18 years and older who have residual low frequency hearing sensitivity and severe to profound high-frequency sensorineural hearing loss, and who obtain limited benefit from appropriately fitted bilateral hearing aids.
Candidates range from normal to moderate hearing loss in the low frequencies (thresholds better than 60 dB HL up to and including 500 Hz). Severe to profound mid to high-frequency hearing loss (threshold average of 2000, 3000, and 4000 Hz ≥75 dB HL) in the ear to be implanted.
The CNC word recognition score for the ear to be implanted must be between 10% and 60%, inclusively, in the ear to be implanted in the preoperative aided condition. The contralateral ear’s CNC score criteria are equal to or better than that of the ear to be implanted, but not better than 80% correct. Moderately severe to profound mid- to high frequency hearing loss (threshold average of 2000, 3000, and 4000 Hz ≥ 60 dB HL) in the contralateral ear.
The cochlear implant system for those 12 to 24 months of age who have bilateral profound sensorineural deafness and demonstrate limited benefit from appropriate binaural hearing aids.
Children 2 years of age or older may demonstrate severe to profound hearing loss bilaterally.
In younger children, limited benefit is defined as lack of progress in the development of simple auditory skills in conjunction with appropriate amplification and participation in intensive aural habilitation over a three to six month period. It is recommended that limited benefit be quantified on a measure such as the Meaningful Auditory Integration Scale or the Early Speech Perception test.
In older children, limited benefit is defined as ≤ 30% correct on the open set Multisyllabic Lexical Neighborhood Test (MLNT) or Lexical Neighborhood Test (LNT), depending upon the child’s cognitive and linguistic skills. A three to six month hearing aid trial is recommended for children without previous aided experience.
CI Contraindications4
A Cochlear Nucleus cochlear implant is not suitable for individuals with the following conditions:
- Deafness due to lesions of the acoustic nerve or central auditory pathway
- Active middle ear infections
- Absence of cochlear development
- Tympanic membrane perforation in the presence of active middle ear disease
Visit our website for more information regarding cochlear implant candidacy and Hybrid candidacy.
References:
-
Gaylor, BA. Raman, G. Chung, M. Cochlear Implantation in Adults. JAMA Otolaryngol Head Neck Surg. 2013 Mar;139(3):265-72.
-
Manrique-Huarte R et al (2016) Treatment for hearing loss among the elderly: Auditory outcomes and impact on quality of life. Audiol Neurootol, 21 Suppl 1:29-35
-
Novak MA, Firszt JB, Rotz LA, et al. Cochlear implants in infants and toddlers. Ann Otol Rhino Laryngol Suppl 2000;185:46-49
-
Cochlear™ Nucleus® CI532 cochlear implant with Slim Modiolar electrode Physician’s Guide. March 2016.