What you’ll learn:
- Why and how the Cochlear™ Care Consensus was developed and why it matters
- Key recommendations across five clinical content areas
- How to consider implementing these recommendations into your care practice
Estimated reading time is 15 minutes.
In this Q&A, Dr. René Gifford, Chief of Research and Audiology at Hearts for Hearing, offers an early look at the newly developed Cochlear Care Consensus. She discusses why this collaborative effort matters, what it means for clinical decision-making, and how it can help patients achieve their full hearing potential.
Q: How have you seen cochlear implant care evolve over your career?
Dr. Gifford: I’ve been a practicing audiologist for almost 30 years and have worked almost exclusively in cochlear implants for 22 of those years. During that time, I have had the privilege of witnessing firsthand some remarkable advancements in CI technology, surgical technique, patient outcomes, assessment materials, and expansion of labeled indications.
Q: Given all these advancements you’ve experienced, what prompted the development of the Cochlear Care Consensus?
Dr. Gifford: Despite decades of advancements, audiologic care and long-term management of adult CI patients has remained largely unchanged since the mid-to-late 1990s. When I was in my clinical graduate program in the late 20th century, audiology clinics were routinely scheduling 7-8 programming visits in the first year following surgery. This follow-up schedule arose primarily from extensive, research-based protocols that had been in place since the earliest CI trials (e.g., Parkinson et al., 2002). However, while our research questions have advanced well beyond CI safety and efficacy, our clinical follow-up schedules remain rooted in early trial designs.

We must adapt our clinical programming and assessment approaches to include practices supported by strong evidence for our growing patient population. Indeed, our potential patient population is much larger than those we currently serve, as just 2-13% of adult CI candidates in the US have at least one implant (Sorkin & Buchman, 2016; Nassiri et al., 2022). Though poor CI utilization has many contributing factors, we as audiologists must acknowledge our role in the procedural bottleneck. Recent studies support implementation of streamlined clinical workflow for the typical adult CI recipient. With adherence to evidence-based practices beginning at CI activation, we are observing significantly shorter time to both CI map stability (Biever et al., 2025) and asymptotic speech recognition (Berg et al., 2023; Holcomb & Smeal, 2024).
Q: What is the Cochlear Care Consensus?
Dr. Gifford: The Cochlear Care Consensus is a collection of evidence-based, clinical consensus statements developed specifically for adult recipients of Nucleus® cochlear implants. It provides standardized, clinical recommendations for optimization of audiology care and outcomes for adult CI recipients from activation through long-term follow-up. The clinical consensus statements cover five key content areas: Programming and Objective Measures, Performance Evaluation/Validation, Contralateral Ear Considerations, Patient Management, and Cochlear Tools and Services.
Q: How was the Cochlear Care Consensus developed?
Dr. Gifford: The Cochlear Care Consensus was developed using a modified Delphi process following a similar format to the Minimum Speech Test Battery Version 3 (MSTB-3; Dunn et al., 2024; Strader et al., 2025). A committee of 10 content experts collectively having over 200 years of CI audiology experience were invited to participate. We were mindful to recruit content experts from across the career lifespan with diverse representation from large academic medical centers, smaller not-for-profit clinics, private practices, and the Department of Veterans Affairs (VA). I was honored to serve as the consensus lead overseeing the process of committee construction, group discussion, and consensus statement review. Our Delphi methodologist, Heather Strader, AuD, is a clinical audiologist and researcher having served on the MSTB-3 consensus committee. Finally, we had three manufacturer representatives whose input and expertise were essential to the committee’s success (Weston Adkins, AuD; Barbara Buck, AuD, and Terry Zwolan, PhD).
During this process, content experts completed a comprehensive literature review to gather peer-reviewed evidence guiding postoperative clinical practice for adult CI recipients. Following literature review, the experts developed consensus statements based on the published literature and presented to the full committee. After two rounds of anonymous voting using a 9-point Likert scale, 57 statements were included in the consensus. Committee members were encouraged to devise clinically feasible protocol recommendations, moving away from our field’s historical practice of applying research-intensive protocols to everyday clinical care.
Q: What are some of the key recommendations that emerged from this process?
The answer to this question could fill a book! But here’s a relatively brief overview of the priority recommendations for the five content areas:
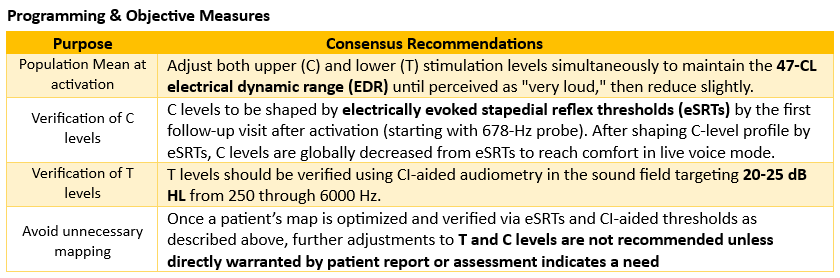
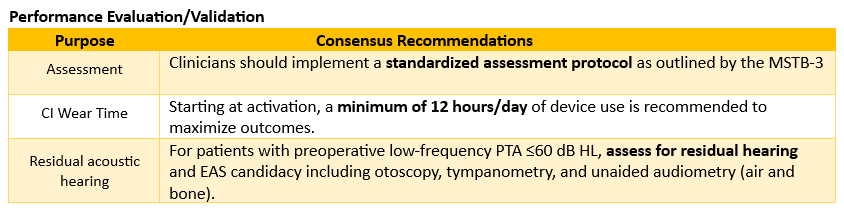



Q: How can the recommendations in the Cochlear Care Consensus help clinicians feel confident that every patient achieves the best possible hearing outcomes?
Dr. Gifford: The Cochlear Care Consensus was developed with the goal of enhancing clinician confidence in clinical decision-making. Its foundation is evidence-based objectivity, where recommendations emphasize quantitative measures such as eSRT, CI-aided thresholds, and standardized speech recognition testing over subjective programming approaches like behavioral loudness scaling. This ensures data-driven precision in clinical decision-making, CI programming, and long-term patient management.
The Cochlear Care Consensus provides clinicians with clear benchmarks and timelines that define optimal outcomes, making it easier to identify when additional intervention is needed. For instance, the recommendations establish specific targets such as 20 to 25 dB HL for aided thresholds, 12 hours of daily wear time, and 56-65% word recognition by 3 to 12 months. These benchmarks remove ambiguity from the care process and help clinicians track progress systematically.
Importantly, these standards afford rapid map stability with results equivalent or superior to traditional behavioral methods while requiring fewer time-intensive appointments. This streamlined efficiency optimizes the care pathway so clinicians can achieve excellent outcomes more effectively. Following the Cochlear Care Consensus recommendations also helps clinicians recognize when maps are already optimized and unnecessary adjustments should be avoided; this consideration parallels clinical optometry research showing that over-refinement based on subjective feedback leads to poorer outcomes (Hughes et al., 2024; Beesley et al., 2025).
Finally, the recommendations provide a systematic framework for problem-solving when patients may not be achieving expected outcomes, including use of remote support tools like Remote Check and encouraging evaluation of contributing factors such as wear time, etiology, and aural rehabilitation participation before assuming device or mapping issues.
Q: Which Cochlear tools and services have been most valuable in your practice, and how do you typically integrate them into patient care?
Dr. Gifford: There are many effective Cochlear tools that assist clinicians in their pursuit of providing exceptional clinical care while also ensuring all patients achieve their own best auditory potential. The two tools and services that have been of greatest value in my own practice have been Population Mean mapping and Remote Check.
| Population Mean | Establishes a common starting point for evidence-based clinical care, reduces unnecessary programming variability, and ensures that all patients can benefit from evidence-based best practices. Note that Population Mean mapping does not impose a one-size-fits-all approach to a clinically diverse patient population. Rather, population mean mapping provides a more efficient activation experience, allowing patients to hear live speech almost immediately without subjective rating of beep-like sounds. |
| Remote Check | Effectively triages patients’ hearing concerns and eliminates unnecessary clinic visits, including routine programming appointments for patients in the maintenance phase of postoperative audiology care. Remote Check includes clinician selected options for assessing physical and functional outcomes including visualization of patient’s postauricular region including incision, data logging, electrode impedances, CI-aided detection thresholds, and speech recognition in noise. Remote Check provides comprehensive and standardized assessment of a patient’s device and performance while also improving access for patients with geographical, transportation, or other scheduling barriers. |
Q: How can audiologists begin integrating these recommendations into everyday clinical practice?
Dr. Gifford: The key to successful integration of the Cochlear Care Consensus is recognizing that these recommendations represent a much-needed shift toward evidence-based objective, standardized, and efficient clinical care that is specifically designed to optimize hearing outcomes for all patients.

The easiest implementation would be with newly implanted patients for whom we can begin with population mean mapping at activation followed by counseling on the importance of 12+ hours of daily processor use. Individualized programming optimization would begin at the next follow-up visit (1 week to 1 month) including eSRT-guided C levels and a CI-aided audiogram to verify that T levels are set to allow audibility of low-level stimuli, such as soft speech. Next, the MSTB-3 protocol is easily implemented as it provides step-by-step guidance on assessing patient outcomes, including specific recorded stimuli, recommended listening conditions (including assessment of non-implanted ears for CI candidacy), and testing time points. Finally, newly implanted patients without preconceived expectations regarding programming and follow-up will more readily accept Remote Check as part of routine maintenance.
Embracing the Future
The Cochlear Care Consensus represents an opportunity to lead with vision and purpose. By embracing evidence-based protocols that balance efficiency with excellence, clinicians can expand access without sacrificing personalized, high-quality care. These guidelines reflect a commitment to continuous improvement, recognizing that serving more patients well requires innovation and collaboration. Whether you’re just beginning to explore protocol changes or have already implemented streamlined approaches, the Cochlear Care Consensus offers validated, expert-endorsed guidance for your journey. Together, we can ensure every eligible patient accesses the life-changing benefits of cochlear implantation through care pathways that are efficient and deeply effective.
Join us at the upcoming March panel webinars to learn practical strategies for bringing the Cochlear Care Consensus into your clinic and taking this important next step forward. Register here.
If you missed the introductory webinar on the Cochlear Care Consensus, you can view the recording in the Cochlear Learning Center.*
* A final review of the statements was conducted after the introductory webinar. As a result, the finalized total is 57 consensus statements, rather than 56 as stated in the webinar recording.

René Gifford, PhD, is Chief of Audiology and Research at Hearts for Hearing in Oklahoma City as well as co-CEO of the clinical board of directors for the Institute for Cochlear Implant Training (ICIT), and Director of the ICIT Advanced Audiology Course (AAC). Her research has been NIH funded for over 20 years and focuses on combined electric and acoustic stimulation (EAS) with particular emphasis on speech & music perception, spatial hearing, and development of speech, language, and literacy for children with hearing loss. She has authored over 170 peer-reviewed publications, two books, and multiple book chapters in the field of cochlear implants and auditory (re)habilitation.
References
- Beesley J, Davey CJ, Elliott DB. (2025). Subjective refraction and prescribing styles used by UK optometrists. Ophthalmic Physiol Opt, 45(5): 1113-1125. doi: 10.1111/opo.13495.
- Berg KA, Holder JT, Gifford RH. (2023). Development of an Optimized Protocol for Cochlear Implant Care to Increase Cochlear Implant Access. Otol Neurotol, 44(8):e635-e640. doi: 10.1097/MAO.0000000000003968.
- Biever A, Bishop GA, Lupo JE, Kelsall DC. (2026). Assessment of Population Mean MAPs for Activation of Cochlear Implant Sound Processors Compared With Behavioral Programming. Otol Neurotol, 47(1):e31-e37. doi: 10.1097/MAO.0000000000004630.
- Dunn CC, Zwolan TA, Balkany TJ, Strader HL, Biever A, Gifford RH, Hall MW, Holcomb MA, Hill H, King ER, Larky J, Presley R, Reed M, Shapiro WH, Sydlowski SA, Wolfe J. (2024). A Consensus to Revise the Minimum Speech Test Battery-Version 3. Am J Audiol, 33(3):624-647. doi: 10.1044/2024_AJA-24-00008.
- Holcomb MA, Smeal MR. (2024). How to Teach an “Old Dog” New Tricks: Improving Clinical Efficiency in a Well-Established Cochlear Implant Program. Otol Neurotol, 45(10):e735-e742. doi: 10.1097/MAO.0000000000004300.
- Hughes AR, Bullimore M, Elliott D. (2024). ‘Ease of adaptation’ predicts preferred spectacle prescriptions better than visual acuity: a retrospective analysis. Clinical and experimental Optometry, 108(1): 79-86. doi: 10.1080/08164622.2024.2304060.
- Nassiri, A. M., Sorkin, D. L., & Carlson, M. L. (2022). Current estimates of cochlear implant utilization in the United States. Otology & Neurotology. https://doi.org/10.1097/mao.0000000000003513
- Parkinson AJ, Arcaroli J, Staller SJ, Arndt PL, Cosgriff A, Ebinger K. (2002). The Nucleus 24 Contour cochlear implant system: adult clinical trial results. 23(1 Suppl):41S-48S. doi: 10.1097/00003446-200202001-00005.
- Sorkin, D. L., & Buchman, C. A. (2016). Cochlear implant access in six developed countries. Otology & Neurotology, 37(2), e161–e164. https://doi.org/10.1097/MAO.0000000000000946
- Strader HL, Dunn CC, Zwolan TA. (2025). Clinical Adaptation of the Minimum Speech Test Battery-Version 3. Am J Audiol, 34(3):450-466. doi: 10.1044/2025_AJA-25-00066.
ꝉ Remote Check for Nucleus Sound Processors is approved for all ages, however certain tests are not suitable for ages below 6. Remote Check does not replace clinical care and does not involve remote programming of the sound processor. Remote Assist for Nucleus sound processors is approved for ages 6 and older. Remote Check and Remote Assist features are only visible and accessible if they are enabled by a clinician. Clinicians should consider the suitability of the feature before enabling Remote Check and Remote Assist. Only available at clinics that have enrolled in Remote Care. For sound processor and app compatibility information visit: www.cochlear.com/compatibility.
Views expressed are those of the individual. Consult your hearing health provider to determine if you are a candidate for Cochlear technology. Outcomes and results may vary.
The content of this guideline is intended as a guide for information purposes only and does not replace or remove clinical judgment or the professional care and duty necessary for each specific recipient case. Clinical care carried out in accordance with this guideline should be provided within the context of locally available resources, expertise, and standards of care and practice.

