You may still have many patients in your clinic who use older Baha® technology—including the Baha 5 and Baha 5 Power which are no longer supported by Cochlear. This article will review two clinically supported pathways that could help ensure your patients are in the most up-to-date technology before they come in requesting a repair on a processor that is no longer supported while also improving their hearing outcomes. Whether they’re active users in need of replacement technology or have stopped wearing their device due to complications, there may be alternate solutions worth discussing.
Option 1: Upgrading to the Baha® 7 Sound Processor
For patients who are still using a Baha 5 Sound Processor or Baha 5 Power Sound Processor, the Baha 7 Sound Processor offers an upgrade with no need for an additional surgery and substantial benefits:
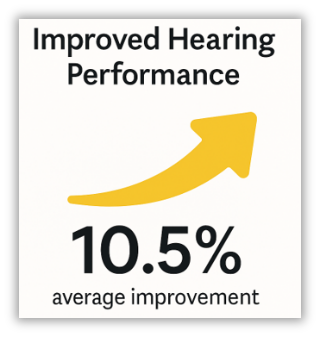
In a clinical study, Baha Connect System users with conductive and mixed hearing loss demonstrated a significant improvement in speech understanding in quiet with the Baha 7 Sound Processor compared to the Baha 5 Sound Processor.1,**
Users also demonstrated significant improvement in speech understanding in noisy environments.1, 2, **
- Wider Frequency Bandwidth
In another study, participants showed a significant improvement in hearing extended high-frequency sounds with the Baha 7 Sound Processor compared to the Baha 5 Sound Processor and Baha 5 Power Sound Processors.1
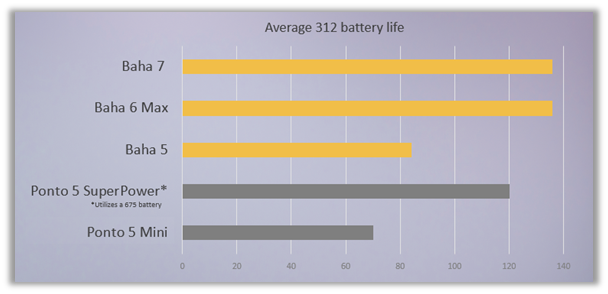
- Unmatched Battery Life4,10-13
- Bluetooth LE Audio & Auracast™ꝉ: The Baha 7 Sound Processor is the first bone conduction processor to provide your patients with direct streaming from public venue broadcasts, Bluetooth LE Audio Accessories (ie. Multi-Mic+ and TV-Streamer+) and continued direct streaming from compatible Apple and Android devices.4,5
These enhancements can make a meaningful difference in daily listening environments, especially for patients who rely on their device in noisy or dynamic settings. It is also a seamless transition for your patient to gain access to better hearing without another surgery – simply email upgrade@cochlear.com with your patient’s information to start the upgrade process!
Option 2: Transitioning to the Cochlear Osia® System

For patients who have discontinued use of their Baha 5/Baha 5 Power Sound Processor, possibly due to skin complications or poor outcomes, transitioning to the Osia System may be an ideal alternative. Benefits of the Osia System compared to a surgical Baha System can include improved comfort and cosmetic appeal, without the same abutment-based skin maintenance and hygiene concerns, while also delivering stable6,7, consistent and powerful sound transmission.8,9
A recent prospective study titled “Clinical and surgical safety in patients transitioning from percutaneous to transcutaneous active bone conduction implants” reviewed 23 cases (18 patients) transitioning from percutaneous to active transcutaneous bone conduction implants (Osia).
Key findings:
- No major complications and only minor adverse events in 5.3% of follow-up points
- Stable audiological outcomes: No significant difference in speech understanding between percutaneous and transcutaneous devices (HINT fixed noise: −0.011%, p = 0.99)
- Quality of Life Improvements (measured using the Glasgow Benefit Inventory***):
- General health: +29.9
- Social support: +31.8
- Physical well-being: +10.6
- Overall GBI score: +27.0 ± 33.9
Surgical Safety
The transition was performed as a single-stage procedure, even in previously contaminated surgical fields. The study highlighted surgical recommendations, including:
- Removal of all skin and subcutaneous tissue around the abutment
- Chlorhexidine irrigation
- Strategic incision placement to avoid scar tissue
These findings support the safety and feasibility of transitioning to the Osia System, especially for patients with a history of skin complications or device intolerance.
Patient-Centered Approach
Not every patient will benefit from a transition to the Osia System, and not every Baha 5/Baha 5 Power Sound Processor user needs to upgrade. The decision must be guided by:
- Patient history and current device use
- Audiological performance and expectations
- Surgical candidacy and risk factors
- Collaborative discussion between audiologist and surgeon

The data supports both pathways as viable options, while emphasizing the importance of individualized care. Audiologists play a pivotal role in helping patients navigate these choices, balancing technological advancements with personal needs.
Consider reviewing your patient database to identify individuals who:
- Are still using the Baha 5/Baha Power Sound Processor and may need replacement technology. Cochlear can help provide visibility to your Baha System recipient base. Please reach out to your local Recipient Solutions Manager to start the discussion.
- Have discontinued use due to complications and may be candidates for Osia.
As technology and patient needs evolve, so do the clinical pathways available to support better hearing outcomes. Whether upgrading to the Baha 7 Sound Processor or transitioning to the Osia System, these options offer meaningful improvements without compromising surgical efficiency or patient comfort. By proactively identifying candidates in your practice, you can help ensure timely access to supported technology and reinforce your commitment to personalized, high-quality care. Find out more about the Baha 7 Sound Processor and the Osia System, visit our website under the “Products and Candidacy” tab.
Get the latest clinical insights, product updates and audiology innovations delivered straight to your inbox. Subscribe to ProNews and be the first to know about new technologies, research breakthroughs and tools to elevate patient care.
*Utilizes a 675 battery
**Based on the Baha 6 Max Sound Processor, using the same technology as Baha 7 Sound Processor
***The Glasgow Benefit Inventory (GBI) is a validated questionnaire used to measure changes in quality of life following a medical intervention, such as surgery. It includes 18 questions across three areas: general health, social support, and physical well-being. Responses rated on a 5-point scale. Scores range from −100 (worst outcome) to +100 (best outcome), indicating perceived changes in quality of life post-intervention.
ꝉ As Bluetooth LE Audio compatible devices become available, a sound processor firmware update will be required to use certain features. Auracast™ broadcast audio capability is subject to third party adoption of the Auracast protocol. The Bluetooth® and Auracast™ word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Cochlear is under license.
- Hua H. Baha 6 Max Home test CIR CBAS5779. Cochlear Bone Anchored Solutions AB, Sweden. 2021; D1801512.
- Hua, H., Goossens, T. and Lewis, A., 2021. Increased maximum power output may improve speech recognition with bone conduction hearing devices. International Journal of Audiology, pp.1-8. https://www.tandfonline.com/doi/full/10.1080/14992027.2021.1959953″
- Davidsson B. Technical Report: Battery autonomy in Baha 6 Max vs Baha 5. Cochlear Bone Anchored Solutions AB, Sweden. 2021; D1770958.
- Hagelbäck K. Baha 7 Sound Processor Connect Datasheet. Cochlear Bone Anchored Solutions AV, Sweden 2024; D2149945.
- FDA Announcement for Baha 7 Sound Processor. Cochlear Americas. 2025.
- Clinical Investigation Report 24-month final analysis. Cochlear Limited, Australia. CBAS5793
- Clinical Investigation Report 6-month follow up.Cochlear Limited, Australia. CBAS5751 (D1703580)
- Dotevall M. Technical Report: Available Gain in Osia vs Baha 5 Power. Cochlear Bone Anchored Solutions AB, Sweden. 2019; D1664198.
- Goh J. OSI200 Implant Accelerated Life Test Report. D1439967. Cochlear Bone Anchored Solutions AB, Sweden
- Ponto 5 Mini Product Information. Oticon Medical. 232708US. 2021
- Ponto 5 SuperPower Product Information. Oticon Medical. 244495US. 2021
- Leung B. Baha 6 Max Connect Datasheet. Cochlear Bone Anchored Solutions AB, Sweden. 2021; D1760797.
- Land J. Baha 5 Sound Processor Connect System Datasheet. Cochlear Bone Anchored Solutions AB, Sweden. 2019; 630908.
This blog is intended to serve as a resource for clinicians to help keep up to date with current clinical literature and is intended for professionals only. Clinical literature is based on research, which may include the experimental use of new or currently available products and technologies. Therefore, literature presented on this blog may represent use of Cochlear products that does not align with the intended use or indications approved by regulatory bodies, also known as off-label use. Cochlear does not condone any off-label use of its products, and it is not Cochlear’s intent to promote off-label use by providing this blog as a resource for healthcare professionals.


